Prosthetic devices are being extensively used to replace or support the function of a infirm or damaged body part. The global cardiovascular prosthetic devices market is expected to rise at a CAGR of 8.5% during the forecast period 2016-2023. Cardiovascular prosthetic devices are also used to repair the diseased or damaged natural valves of the heart. Hence, this has led to increased adoption of cardiovascular prosthetic devices for the repairment of damaged heart valve and for the treatment of patient suffering from septal defect. According to Centers for Disease Control and Prevention, conal septal defect is more prevalent in Asians while membranous septal defect is found in 70% patients of septal defect and atrioventricular septal defect, is found in approximately 5% septal defect patients.
The key factor responsible for the growth of global cardiovascular prosthetic devices market is the increasing prevalence of cardiovascular diseases. The prevalence of cardiovascular diseases (CVD) such as ischemic heart disease, heart failure, strokes etc. incidence are rising across globe leads to increase in the demand of cardiovascular prosthetic devices as it helps in the treatment of cardiovascular diseases. According to European Heart Network, in Europe, there were approximately 11.3 million new cases of CVD every year and 6.1 million new cases of CVD in 2015. In Europe, every year more than approximately 85 million people suffered with CVD and approximately 49 million people suffered with CVD in 2015. The main cause of death in Europe was CVD and approximately 45% people died due to CVD out of all deaths in 2015. According to Center of Disease Control and Prevention, approximately 28.4 million adults were diagnosed heart disease in 2015 in America. In the U.S. approximately 5.7 million adults suffered from heart failure in 2015. Heart failure was the major cause of death in America in 2015 with 1 out of every 9 deaths was due to heart failure. Approximately half of people who suffered from heart failure die within the 5 years of diagnosis. The total healthcare expenditure due to heart failure in the U.S was estimated to be approximately $30.7 billion each year. Furthermore, According to Center of Disease Control and Prevention, cardiovascular disease was considered as the main cause of death and approximately 801,000 deaths occurred in America in 2016 i.e. 1 of each 3 deaths in the US is due to cardiovascular diseases. In 2016 approximately 2,200 deaths in US are due to cardiovascular disease every day; it is an average of 1 death in every 40 seconds. According to Center of Disease Control and Prevention, the death rate due to cardiovascular diseases every year was more than other diseases such as cancer and chronic lower respiratory disease as per Center of Disease Control and Prevention.
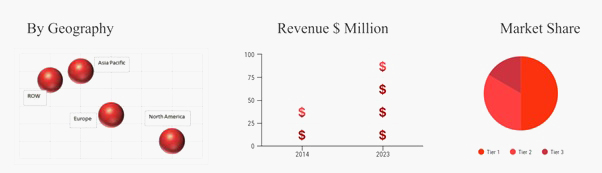
Source: OBRC Analysis.
The report on global cardiovascular prosthetic device market includes type and end users.
Types of cardiovascular prosthetic device include:
- Cardiac Prosthetic Devices
- Vascular Prosthetic Devices
The end users of cardiovascular prosthetic device include:
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers (ASCS)
The report scope is widely categorized on the basis of its types which include Cardiac Prosthetic Devices and Vascular Prosthetic Devices.
The global cardiovascular prosthetic device market report has been geographically segmented in:
- North America (U.S. & Canada)
- Asia Pacific (China, India, Japan, RoAPAC)
- Europe (UK, France, Germany, RoE)
- Rest of World
Geographically, North America is the largest market region for global cardiovascular prosthetic device market in terms of market revenue share. The key factors driving the market growth in North America is the rising prevalence of cardiovascular diseases in U.S. Moreover, according to Silverbook, every year, approximately 5 million people in North America are diagnosed with heart valve disorders. Furthermore, increasing adoption rate of cardiovascular prosthetic devices along with rising geriatric population and the unhealthy lifestyle of the young generation is expected to propel the growth of market. However, Asia Pacific is expected to emerge as the fastest growing market region owing to the increasing geriatric population. As per the Help age organization, 3.8% of the population in India is found to be above the age of 90.
Global cardiovascular prosthetic device market report covers segmentation analysis of type, applications and end users. Report further covers segments of types of cardiovascular prosthetic devices which include Cardiac Prosthetic Devices and Vascular Prosthetic Devices. Cardiac prosthetic devices such as stents and prosthetic heart valves are used to maintain the proper functionality of the heart. Stents are used to maintain the blood flow, and also helps in the reduction of blockage in the arteries, whereas prosthetic heart valves are being extensively adopted by patient suffering from heart valvular disease or dysfunctional heart valve. Cardiac prosthetic devices are the leading type segment owing to its increased adoption due to rising cardiac disorders. Cardiac prosthetic devices can be further categorised into prosthetic heart valves, annuloplasty rings, defibrillator leads, pacemaker leads, tissue patches, artificial hearts and ventricular assist devices. The end users of global cardiovascular prosthetic devices include Hospitals, Specialty Clinics and Ambulatory Surgical Centers (ASCS).
The major market players of the global cardiovascular prosthetic device market are:
- ABBOTT LABORATORIES
- BIOSENSORS INTERNATIONAL
- COLIBRI HEART VALVE
- INSPIREMD
- MEDTRONIC PLC
- OTHERS
These companies using various strategies such as merger & acquisition, collaboration, partnership and product launch. Whereas, product and service launch is the key strategy adopted by the companies in the cardiovascular prosthetic device market.
For Example; In April 2016 Abbott Laboratories had announced a definitive agreement to acquire St. Jude Medical to expand its customer base and strengthen its product offerings.
In 2015, Medtronic PLC had launched Freestyle bioprosthesis, for the replacement of prosthetic aortic valves or malfunctioning native as an alternative option of aortic root replacement.
The report covers detailed analysis of companies which comprises overview, SCOT analysis, product portfolio, strategic initiative, strategic analysis, competitive landscape and market share analysis in cardiovascular prosthetic device market.
Key reason to buy the report:
- The report includes market estimation, forecast and analysis for the year 2016-2023
- Report includes detailed analysis of different segments such as type, applications and end users of cardiovascular prosthetic device.
- Identify and understand the strength, opportunities, challenges and threat of the cardiovascular prosthetic device market.
- Covers details analysis of Porters 5 force model and other strategic models and also covers revenues, market share analysis, and competitive landscape analysis of major players of cardiovascular prosthetic device market.
- Detailed analysis of various the regulatory policies which are affecting the global cardiovascular prosthetic device market.
How we are different from others:
At Occam’s we provide an extensive portfolio which is comprehensive market analysis along with the market size, market share, and market segmentations. Our report on global cardiovascular prosthetic device market offers detailed analysis of strategic models such as investment vs. adoption model, see saw analysis and others strategic models. Also, the report contains the detailed analysis of application, adoption scenario and decision support for each segment. The report discusses competitive landscape of the cardiovascular prosthetic device market, with giving extensive SCOT analysis of key companies.
Key findings of the global cardiovascular prosthetic device market:
- The key factor responsible for the growth of global cardiovascular prosthetic devices is the increasing prevalence of cardiovascular diseases.
- North America is the largest market region for global cardiovascular prosthetic device market in terms of market revenue share.
- Cardiac prosthetic devices are the leading type segment owing to its increased adoption due to rising cardiac disorders.
- Product and service launch is the key strategy adopted by the companies in the cardiovascular prosthetic device market.


