Intrauterine contraceptive device is a small T-shaped device which is inserted in the uterus of a woman in order to prevent pregnancy. Intrauterine contraceptive devices are a form of long lasting reversible method for birth control. They are 99% effective in preventing unwanted pregnancy, convenient to use, are reversible and starts working immediately after being inserted in the uterus. Due to these advantages, intrauterine contraceptive devices are used by large number of women across the world. Global intrauterine contraceptive device market is expected to rise at a CAGR of 10% during forecast period 2016-2023. Due to increasing number of abortion cases, unplanned pregnancy, reduction of birth control rate has led to increased adoption of intrauterine contraceptive devices. Moreover, there has been an increment in the number of copper intrauterine contraceptive devices users from 0.25% to 0.3% from 2011 to 2016. Furthermore, decrement in the sterilization rates had led to the increase in intrauterine contraceptive devices insertion rates, thereby suggesting that increasing numbers of women adopted for reversible methods of long term contraception instead of permanent sterilization.
Key factors driving the market growth is the increasing prevalence of sexually transmitted diseases. Sexually transmitted diseases have always been a subject for concern due to the unhygienic sexual activities condition and negligence among the people regarding the precautions. According to U.S. based Centres for Disease Control and Prevention, in 2016, large numbers of cases of sexually transmitted diseases such as gonorrhoea, syphilis, chlamydia etc. were recorded in United States. Moreover, untreated sexually transmitted diseases can lead to several health complications such as infertility, sterility, pre-term delivery, genital neoplasia, and foetal/neonatal pathologies. Furthermore, number of cases of syphilis increased to 19% in 2016 thereby causing stillbirths and infant deaths, as reported by public health officials. Also, chlamydia is the most common STD with over 1.5 million cases globally in 2016. According to the 2016 report of World Health Organization, more than 1 million people gets infected by Sexually Transmitted Infections (STIs) every day worldwide. Moreover, sexually transmitted infections like syphilis and herpes can increase the risk of HIV. Therefore, intrauterine contraceptive devices are increasingly adopted to reduce the risks of sexually transmitted diseases and pelvic inflammatory disease. However, intrauterine contraceptive devices do not prevent STD completely, but reduces its risk to a significant extent.
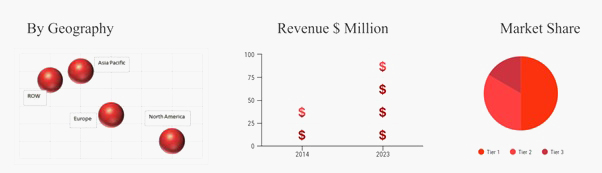
Source: OBRC Analysis.
The report on global intrauterine contraceptive device market includes device type and products.
Types of intrauterine contraceptive device included are:
- Hormonal intra uterine device
- Copper intra uterine device
Products of intrauterine contraceptive device included are:
- Mirena
- Skyla
- Paragard
- Essure
- Levosert
- Others
The report scope is widely categorized on the basis of its types which include hormonal intra uterine device and copper intra uterine device.
Global intrauterine contraceptive device market report has been geographically segmented in:
- North America (U.S. & Canada)
- Asia Pacific (China, India, Japan, RoAPAC)
- Europe (UK, France, Germany, RoE)
- Rest of World
North America is the largest market region for intrauterine contraceptive device market owing to the increased adoption of these devices in United States. There is high rate of unplanned pregnancies among the teenagers in the U.S. Moreover, the increasing number of initiatives taken by the U.S. government has helped to reduce the rising number of unplanned pregnancies and abortions rate in the United States. Furthermore, many U.S. based private companies have also contributed and provided the solutions for issues during pregnancy which includes teenage pregnancies, maternal mortality, increasing number of abortions etc., by promoting the advantages and use of intrauterine contraceptive devices. Furthermore, Asia Pacific is anticipated to emerge as the fastest growing market region owing to the presence of leading pharmaceutical companies in Asia Pacific countries such as China, Japan, India etc.
Global intrauterine contraceptive device market report covers segmentation analysis of device type and products. Report further covers segments of device type which includes hormonal intra uterine device and copper intra uterine device. Among these segments, copper intra uterine device is the leading segment due to its increased adoption as it is more convenient to use, low threats of side effects, and is considered to be safe for women. Among the females using IUD, 79% of them use copper intra uterine device worldwide. Report further covers segments of products such as Mirena, Skyla, Paragard, Essure, Levosert etc. Mirena is the widely used product thereby accounts for highest market growth. Mirena intrauterine contraceptive device with Progestogen is extensively used to facilitate long time birth control and is 99% effective than any other product.
The major market players of the global intrauterine contraceptive device market are:
- AGILE THERAPEUTICS
- ACTAVIS PLC
- BAYER HEALTHCARE AG
- TEVA PHARMACEUTICALS INDUSTRIES
- JOHNSON & JOHNSON
- OTHERS
These companies using various strategies such as merger & acquisition, collaboration, partnership and product launch. Whereas, product and service launch is the key strategy adopted by the companies in the intrauterine contraceptive device market.
For Example: In September 2016, Bayer Healthcare AG launched its new intrauterine contraceptive device product, named Kyleena intrauterine device for birth control.
In September 2016, Allergan and Medicines 360 announced the launch of their new intrauterine contraceptive device named LILETTA which would prevent pregnancy up to 3 years.
The report covers detailed analysis of companies which comprises overview, SCOT analysis, product portfolio, strategic initiative, strategic analysis, competitive landscape and market share analysis in intrauterine contraceptive device market.
Key reason to buy the report:
- The report includes market estimation, forecast and analysis for forecast period 2016-2023.
- Report includes detailed analysis of different segments such as equipment type and applications of intrauterine contraceptive device.
- Identify and understand the strength, opportunities, challenges and threat of the intrauterine contraceptive device market.
- Covers details analysis of Porters 5 force model and other strategic models and also covers revenues, market share analysis, and competitive landscape analysis of major players of intrauterine contraceptive device market.
- Detailed analysis of various the regulatory policies which are affecting the global intrauterine contraceptive device market.
How we are different from others:
At Occams we provide an extensive portfolio which is comprehensive market analysis along with the market size, market share, and market segmentations. Our report on global intrauterine contraceptive device market offers detailed analysis of strategic models such as investment vs. adoption model, see saw analysis and others strategic models. Also, the report contains the detailed analysis of application, adoption scenario and decision support for each segment. The report discusses competitive landscape of the intrauterine contraceptive device market, with giving extensive SCOT analysis of key companies.
Key findings of the global intrauterine contraceptive device market:
- The global intrauterine contraceptive device market is expected to rise at a CAGR of 10% during the forecast period 2016-2023.
- North America is the largest market region for intrauterine contraceptive device market.
- Copper Intra uterine device is the leading segment due to its increased adoption.
- Product and service launch is the key strategy adopted by the companies in the intrauterine contraceptive device market.


